Caustic Cleaners: Applications and Formulation Challenges
When it comes to "Heavy Duty Cleaning," there are few fast-acting and effective options available when developing a formulation. It's rare to find ingredients as reactive as alkalis/hydroxides, such as sodium hydroxide, also known as caustic soda, or strong acids, such as hydrochloric acid, also known as muriatic acid.
.png)
While these compounds certainly offer excellent cleaning performance, their acidic or alkaline nature distinguishes them in terms of their cleaning function. In this article, we will focus on highly alkaline products, also known as caustics.
To understand the function of a strongly alkaline or strongly acidic component, read the article on the influence of pH on cleaning, click here.
Sometimes it is hard to avoid using caustic agents for efficient, quick, and inexpensive cleaning. But what makes these ingredients—primarily caustic soda (sodium hydroxide) and then potash (potassium hydroxide)—so effective?
Well, they are highly reactive and electrolytic compounds, and this gives them the following characteristics and behaviors when it comes to the cleaning process:
- Saponification (soap formation): Once they come into contact with fat or some more acidic proteins, they react to form soap. Soap, in turn, is soluble and can therefore be easily rinsed away with water.
- Corrosivity: Its reaction with metal ions sometimes causes a stripping process, or the removal of a metal film. This gives the impression that the product has "renovated" the surface, when in reality it has corroded it.
- Degradation of organic materials/polymers:It has the ability to react with organic materials, degrading them into smaller particles. This makes these organic materials much more malleable and easier to remove. A well-known example is the use of caustic soda as a paint remover, for example. It reacts with the paint and degrades it. In this process, it is easy to see the wrinkled and soft paint. Therefore, it is easier to remove with a spatula, without applying too much force.
- Dispersion of solids:Its ability to degrade organic matter, combined with electrolytic force, allows the solids present in the cleaning bath, both organic and mineral, to remain suspended for longer, facilitating rinsing. The degraded organic materials become much smaller particles, facilitating their suspension, and the minerals present in the same wash, due to the electrolytic force of caustic soda, for example, interact with each other at an intermolecular level, forming a large network of molecules, some attached.
Trying to bring it to a more practical level, let's discuss some situations in which the presence of these ingredients is quite common:
Automotive Cleaning

Less aggressive and more environmentally friendly formulations do indeed exist. However, these options are still very expensive compared to caustic formulations and, therefore, are only used in certain niches of this market.
In this type of cleaning, the most common ingredient is sodium hydroxide. It is unlikely you will find another compound as effective at cleaning truck chassis, wheels, and tires, for example.
What stands out here is its dispersion and corrosiveness, allowing it to extremely efficiently remove this characteristic road grime, which we call Traffic Film. This complex mixture of residues containing asphalt, soil, and burnt engine oil, mixed with a complex array of dirt carried by rain and wind, and subsequently dried by the sun.
This type of dirt has a strong adhesion to the surface, which, without a very strong chemical agent, will require a certain level of scrubbing. The most critical case is tires. In this case, even scrubbing does not solve the problem. What the soda can do is react and "erode" the rubber, allowing the dirt that has penetrated" there and is no longer able to be removed.
It is important to note that, despite its effectiveness and low cost, caustic soda is still widely used in areas it is not recommended for cleaning, such as the mechanical parts of automobiles. These areas contain many aluminum and metal parts that are subject to severe attack and corrosion, as well as electronic components and plastic parts, such as tanks, small parts, and hoses that can easily crack and break.
ion of surfactant
Although soda is the main ingredient in this type of formulation, it alone does not perform as well when combined with suitable surfactants. And that is a new challenge begins.
Not all surfactants are stable in a caustic environment, and not all deliver the best cleaning potential in this type of system.
Because high foaming is generally desired, formulators often use sulfonic acid and/or sodium lauryl ether sulfate as surfactants in this type of formulation. However, these two products have limitations in this type of formulation.
Sulfonic acid becomes insoluble above pH 12. Being insoluble in the formulation, it tends to precipitate (form phases). Furthermore, when diluted for later use, the product takes time to dissolve, and a great part of the surfactant present will not be fully active. To perform its role as a surfactant, it must be dissolved in water. To make sulfonic acid soluble in a caustic medium, a high concentration of hydrotrope is required, adding significant cost to the formulation.
Sodium Lauryl Ether Sulfate is only soluble in caustic media up to a certain caustic soda concentration. The higher the caustic soda concentration, the lower its solubility, and, like sulfonic acid, it will require another hydrotropic ingredient to stabilize the formulation and allow the lauryl to dissolve.
To learn more about Hydrotopes, read the article on this topic.
For cultural reasons, it is also desirable for the product to have viscosity. And, once again, thickeners that are stable against caustic soda are not common. In this case, the best option for generating viscosity in this type of product is to understand the appropriate ratios of the correct surfactants.
Understanding these challenges and difficulties, Macler, through its SmartLab, developed two formulation options for stable, high-foaming, and high-performance Automotive Caustic Degreasers. To access and benefit this knowledge, simply click the banner below:

Industrial Kitchen
.png)
In this kind of environment, grills, griddles, and hoods are always a challenge. High fat content and charred food pose a significant challenge. Here, once again, the dispersion and corrosive properties attack the charred parts very effectively. Greases, on the other hand, are attacked by saponification—the reaction of the alkaline ingredient with the fat, forming soap. Since soap is water-soluble, it can be removed by rinsing.
It is worth noting that, depending on the type of alkaline ingredient used, soaps will be formed that are easier or harder to rinse out. In this case, the more reactive and strong the alkali, the harder the soap will be and, therefore, the harder it will be to rinse out. This doesn't mean the soap is less soluble; it simply becomes more solid, and therefore requires more time to dissolve. Therefore, we can organize the most commonly used ingredients in this type of cleaning in order of the hardness of the soap that will be formed, and, consequently, easier to rinse out:

Knowing this, it is a good idea to use these ingredients together in a formulation. This will make the product more effective, as it will benefit the high reactivity of Soda and Potash, with softer, easier-to-rinse soaps resulting the reaction with Potash and Monoethanolamine.
It is important to note that certain equipment and surfaces should not be cleaned with strongly alkaline products, especially aluminum, which is very susceptible to corrosion this type of compound.
ion of surfactant
Here again, these alkaline components alone do not perform well. A surfactant is essential for them to penetrate deeper into the dirt and pores of surfaces for the formulation to be truly effective. The challenges for formulators are very similar to those mentioned above for an automotive degreaser. ing carefully the surfactant and the rare availability of soda-compatible thickening ingredients become major obstacles when developing an alkaline degreaser and descaler for use in industrial kitchens.
However, after years of research and development into this type of product and application, Macler's SmartLab suggests some extensively tested, stable, and high-performance formulations. For those seeking this type of solution, access to this material is a great shortcut in a formulator's professional career. Years of experience and work condensed into one place. To access, simply click the banner below:

CIP cleaning
.jpg)
CIP (Clean in Place) cleaning is an automated sanitation process used especially in food and beverage production industries to clean equipment and piping without the need for disassembly. The process involves circulating cleaning solutions (detergents and disinfectants) and water at controlled temperatures and flows to remove residue and contaminants.
The steps used in this type of cleaning are usually as follows:
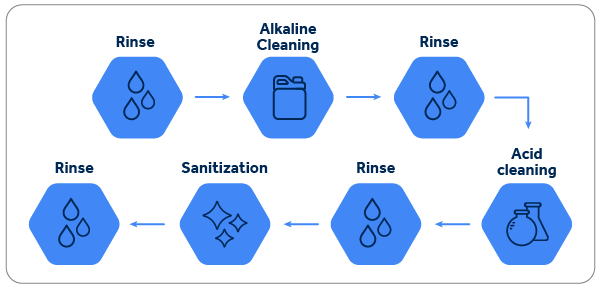
Let's focus on the alkaline cleaning process. The goal of this step is to remove primarily the greasy and protein-based dirt that builds up on the pipe walls over time.
This type of cleaning is a great example of how to understand Sinner's Circle in practice. Since scrubbing is not possible, chemical action, concentration, temperature, and contact time are key factors for the success of this type of process. To better understand Sinner's Circle, read the article. Click here.
Each type of industry has its own specific type of dirt. In the dairy industry, for example, pipes are heavily contaminated with grease, protein, and calcium, making cleaning even more difficult. However, precisely because it's the most reactive alkaline ingredient, caustic soda is the most widely used ingredient in all of them. Its reactivity in this type of process is even stronger because they typically work with heated solutions, which can reach temperatures of up to 90°C during the cleaning process. It's not uncommon, particularly due to the presence of calcium ions, to use tetrasodium EDTA or some other sequestrant in conjunction with caustic soda for enhanced performance.
ion of surfactant
In CIP cleaning, some surfactants can be applied to increase cleaning performance. However, they must be low-foaming products, as foaming can damage the equipment used in this process, especially the pumps.
Although soda alone does not cause foaming, we saw above that it reacts with fats to form soaps. Therefore, during CIP cleaning, soap formation occurs, presenting a high potential for foaming.
Therefore, in addition to choosing a low-foam surfactant, it will be necessary to use an antifoam agent, which must be added as an additive to the cleaning solution at the time
the process begins, along with the Soda, since antifoam agents do not have good compatibility with Soda in high concentrations.
Furthermore, regarding the use of surfactants in this application, they don't perform as effectively as in previous applications. The main function here is to reduce the surface tension of the cleaning solution so it can penetrate deeper into the dirt and potential cracks and grooves in the walls of pipes and equipment, especially in areas there are valves, connections, and welds.
Among some surfactant options, Macler highlights two options its portfolio that are commonly applied in these cases: Isogen SE 32, a product of our own manufacture, and the Berol LFG 61, manufactured by Nouryon, a Dutch multinational distributed by Macler in Brazil. Click the banner below to see Macler's recommended formulation for this application.

It is very difficult to cover all the situations related to caustic formulations in a single text. That is why Macler has a team of professionals in its SmartLab, ready to assist you with any questions or specific development needs. Contact us to find out more.
Learn More
Perhaps one of the best-known chemicals used by Brazilian consumers, which has a dramatic influence on culture and behavior with regard to cleaning, is sodium hypochlorite, commonly known as “bleach.”
When the temperature changes, turbidity, phase separation, crystal formation, or changes in viscosity may occur. Two parameters are critical in this analysis: the fog point and the cloud point.
This article will discuss best practices for peroxide-based formulations and tips for developing a high-performance, cost-competitive, and highly stable product.
Our chemistry
We use our labs to create
intelligent chemical solutions balanced with your reality.
Products
We use our R&D center, our own laboratory with experienced professionals, to deliver intelligent chemical solutions in balance with your reality.