
What exactly is pH, and what is it used for?
One of the most important parameters to control (or at least monitor) when working with chemicals is pH. So, if you work in an industry like this, you have certainly come across these two little letters at some point. But in practice, what does this acronym mean, and how can it impact your products?
.jpg)
Definition of pH
Let's begin to explore this term and see this topic will lead us. Generally speaking, we can start by defining pH. If you ask a chemist this, you are likely to get an answer like "pH is the hydrogen potential of a solution" or "pH is a mathematical scale used to measure the acidity or basicity of a solution."
Note that none of the definitions given above are actually wrong. But note that they also mean little to people without a more in-depth knowledge of chemistry. After all, what are these terms: hydrogen potential, acidity, or basicity? Out of the three terms mentioned, the last two might seem a bit easier to understand, but do the terms acidity and basicity on their own really mean anything?
These two terms do not necessarily convey anything particularly assertive, so we will consider them as vague as potential hydrogen. However, we can use them to try to understand pH more understandably—and to do so, we will first bring one of our senses to the table: taste.
These two terms are easily associated with two types of flavors. Acidity can be linked to sour taste, i.e., foods rich in sour flavour are so-called acidic foods. For example, lemon and other citrus fruits fall into this food group, being a group rich in citric acid. Basicity, or alkalinity, is directly linked to foods that leave a dry, rough, or wrinkled mouthfeel, such as dry wines and under-ripe fruits, meaning an astringent taste.
Now that we have begun to understand this distinction between acidity and basicity, we can start to introduce the concept of pH: if we know these two types of taste exist, wouldn't it be possible to establish some way to measure how sour or astringent a food is? For example, if we compare the taste of a lemon to that of lettuce, we know that lemon tastes much more acidic than lettuce, which has an astringent taste—but how much more acidic is this taste?
We can then introduce pH as a way to measure how acidic or basic a food is. Therefore, if we identify something measurable to quantify acidity, we can establish a scale. However, to know what will be measured, we cannot escape chemical and physical concepts.
Going back in time…
In the late 1800s, Swedish scientist Svante Arrhenius, one of the founders of the science now called physical chemistry, developed the theory of water autoionization while studying a way to explain the conductivity of electrolytes. In his studies, Arrhenius proposed that water molecules in the liquid state do not behave statically, but rather in a dynamic equilibrium, they break down and reform. In chemical terms, Arrhenius proposed that the water molecule, HO, in the liquid state behaves as follows:
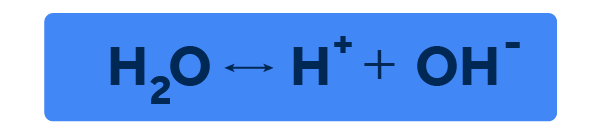
So, according to Arrhenius' proposal, water molecules not only undergo interactions of their internal forces, forming and breaking hydrogen bonds, but also find themselves in this dynamic dance, the molecule breaks, generating positively and negatively charged ions, and reconstitutes itself, returning to the neutral state of a molecule.
Based on this theory, Arrhenius proposed the first theory of acids and bases in 1887, in which he proposed that an acid would be a substance that, when dissolved in water, would ionize and produce a cation H, called hydrogen, which has a positive charge. A base, on the other hand, would be a substance that, when dissolved in water, would ionize and produce an anion OH, called hydroxyl, which has a negative charge.
Although simple, this theory proposed by Arrhenius was somewhat restrictive. Then, in 1923, scientists Johannes Brønsted and Martin Lowry refined Arrhenius's theories, redefining acids as substances capable of donating H+ ions and bases as substances capable of accepting H+ ions. Later came Lewys's proposal of acids and bases, which made the concept more comprehensive and complex, defining acids and bases as compounds capable of donating or accepting electron pairs. This new, broader definition by Lewys allows us to include ammonia, NH, and its organic derivatives (such as amines) as bases, since, although they do not accept H+ in aqueous solution, as proposed by Johannes Brønsted and Martin Lowry, they are substances capable of donating electron pairs.
The pH scale
Based on these definitions of acids and bases, we can then infer that an acidic medium will be one deficient in electrons, and a basic (or alkaline) medium will be one rich in
free electrons. In other words, we've seen that we can assess the acidity or basicity of a given medium based on certain electronic characteristics—and this is the famous pH scale comes in.
pH is nothing more than a numerical scale constructed calculations based on electrical current measurements of known aqueous solutions. The electrical current, which is precisely the movement of electrons, was used as it provided the condition for indicating the "availability" of free electron pairs in those solutions. It was these measurements that it was possible to identify the basicity or acidity of a solution. This scale was first developed in 1909 in Denmark by chemist Søren Lauritz Sørensen at the Carlsberg brewery, and was refined after the dissemination of the Brønsted and Lowry theories.
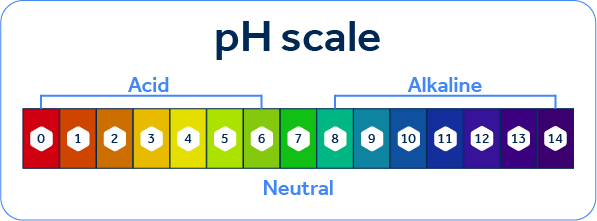
In general, it is very common to hear that the pH scale ranges 1 to 14, with the more acidic a medium, the lower its pH, and the more alkaline, the higher its pH. While the pH in most situations actually falls between these values, some very strong acids and bases can cause the pH to fall below 1 or above 14, but these are very specific cases.
Following the logic of the pH scale values presented, we can even think of it as a kind of scale: if values closer to 1 are acidic and values closer to 14 are basic, what can we say about the region in between these values, that is, the pH value equal to 7? This is the neutrality value of a solution, that is, it is a value on the scale at which we can say that the solution is neither acidic nor basic.

pH and the cleaning products industry
Now that we have defined pH, acids, and bases, what can we say about this topic regarding sanitizing products? After all, what we have seen, any mixture containing water can have a pH value, and water is the most common vehicle in chemical industries in general.
pH, above all, is a fundamental property that is crucial for various classes of cleaning products. To put it simply, an acidic pH will react with dirt or basic surfaces, generally made of minerals.
A basic pH tends to react with acidic dirt or surfaces, such as grease and oil. This makes a big difference because if, for example, an acidic pH is used to clean oils and grease, this will compromise the product's performance, as it will not react with this type of dirt. Products that are used for heavy-duty oil and grease removal tend to benefit a more basic/alkaline pH, as this condition favors the saponification of oils and grease, which is precisely the reaction of the alkaline component of the formulation with the grease, forming soap. Since the soap is water-soluble, the dirt can be removed by rinsing.
Below is a table with examples of surface types and soiling that are more or less suitable for each pH. This knowledge is crucial when the development of a new product starts.
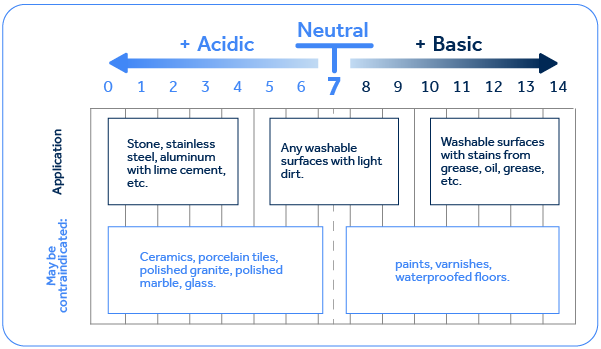
In addition to this benefit for some specific types of cleaning, some substances require specific pH ranges to stabilize. For example, sodium hypochlorite and hydrogen peroxide. Sodium hypochlorite, one of the main sources of active chlorine used in chlorine bleaches, requires an alkaline pH to stabilize. Hydrogen peroxide, on the other hand, requires an alkaline pH if it doesn't have another stabilizing agent, such as DeepSense technology developed by Macler, which needs a more acidic pH to remain stable.
pH is also important when working with certain types of surfactants, such as amine oxides and betaines. Under neutral and alkaline conditions, these surfactants behave as if they were nonionic. However, under acidic conditions, these surfactants exhibit zwitterionic behavior. In acidic conditions, their nitrogen atoms are protonated, generating a positive charge, making them act as cationic surfactants. Knowing whether the components in your formula have this characteristic is essential, as this change in surfactant type can create incompatibilities that lead to instability and even phase separation in the formulation.
Attention to regulations
Another very important point that should draw your attention in this context concerns legal aspects. The National Health Surveillance Agency (Anvisa) imposes pH limits for your product to be registered or even simply notified for sale. In the case of highly acidic products, with a pH of 2 or lower, or highly alkaline products, with a pH of 11.5 or higher, product registration is required, in addition to determining its corrosive potential, for the product to be classified as risk 1 or risk 2, according to RDC No. 59 of December 17, 2010. These extreme situations also require special care with labeling and packaging, as set out in RDC No. 697 of May 13, 2022.
We have seen in this text that pH is a fundamental concept for working in the chemical industry, and understanding the nuances of its different ranges is extremely important—whether for the performance, stability, or even legal status of your product. Even so, it is still possible to discuss pH and its numerous other effects in its formulation.
Do you have any specific questions regarding the pH of your formulation? Contact the SmartLab team so we can help!

Learn More
Perhaps one of the best-known chemicals used by Brazilian consumers, which has a dramatic influence on culture and behavior with regard to cleaning, is sodium hypochlorite, commonly known as “bleach.”
When the temperature changes, turbidity, phase separation, crystal formation, or changes in viscosity may occur. Two parameters are critical in this analysis: the fog point and the cloud point.
This article will discuss best practices for peroxide-based formulations and tips for developing a high-performance, cost-competitive, and highly stable product.
Our chemistry
We use our labs to create
intelligent chemical solutions balanced with your reality.
Products
We use our R&D center, our own laboratory with experienced professionals, to deliver intelligent chemical solutions in balance with your reality.
