Macler provides a detergent formulation guide

Since 1996, when Macler launched Noxipon, a thickener which has changed the way detergents are produced in Brazil, Macler has helped thousands of customers to develop and reformulate their detergents.
Over all these years, Macler has accumulated experience and acquired great expertise in this field. In order to spread this knowledge, the company has created a guide for developers to shorten their journey towards a simple, balanced and cost-effective formulation.
To better understand the indications in this guide, it is important to clarify some beliefs and myths that have been circulating in this market for many years.
Differences between 90% and 96% Sulfonic Acid
Many say that 90% Sulfonic Acid generates greater viscosity than 96% Sulfonic Acid, because the 90% product contains a greater amount of Sulfuric Acid than the 96% product.
However, this statement is neither true nor coherent. If this were the factor, this would be easily corrected by adjusting the salt concentration in the formulation, which does not occur. The reason for this is that the Sulphuric Acid will be neutralized by Sodium Hydroxide during the production of the detergent and will form Sodium Sulphate, a salt commonly used in formulations and does not present any great differential in terms of generating more or less viscosity in the detergent.
Furthermore, 96% Sulfonic acid has a higher cloud point, indicating that, even with a lower concentration of Sulfuric Acid, it is less soluble than 90%. So what makes the two different?
They are manufactured using different synthesis routes, which generate different isomers during their production. In other words, the Sulphonic radical changes its position in the carbon chain of the molecule, and this directly influences the solubility of the molecule and, consequently, the micelle that will form it.
Since viscosity is greatly influenced by the solubility of the ingredients, these differences that we observe in the viscosities of these two products refer to the differences in solubility between them that arise their different isomerisms.
Amount of salt x Detergent foam

It is very common to hear people saying that adding too much salt will harm the formula's foaming. The salt itself does not noticeably interfere with the foam when applied. What can interfere is the quality of the salt used.
Low-quality salts can often be highly contaminated with iron or calcium and magnesium carbonate. Therefore, these cations react with anionic surfactants, inactivating them and impairing the foaming, stability, and general performance of the product. Hence, it is important to use a sequestrant to protect the formulation and guarantee its efficiency.
Volume X Foam quality
One of the main characteristics to look for in a dishwashing detergent is the foam volume. The higher the volume, the better. But is this enough to assess the quality of the foam?
A high volume of foam is not always directly related to its quality. This is because the stability of the foam, in most cases, is even more important than the volume. It is stability that will ensure the product remains on the sponge during use.

Often, the volume of the foam is associated with a large bubble that breaks more easily. Therefore, a more compact foam, with smaller diameter bubbles, becomes more stable, guaranteeing a greater number of dishes washed per gram of detergent.
The assessment of foam quality must then be a cross between its volume and its stability.
Is Sodium Lauryl Ether Sulfate all the same?
It is interesting to know this ingredient, which is so important in the manufacture of various sanitizing and cosmetic products, has some particularities that are not commonly communicated.
Firstly, this product contains an ethoxylated part in its molecule. In Brazil, we mostly use Lauryl with 2 moles of ethoxylation, but there are also those with 1 or 3 moles, which causes significant differences in the viscosity, cleanliness and turbidity of the formula, as they alter the solubility of the molecule.
Another important distinction concerns its source of obtaining. The term Lauryl refers to a 12-carbon chain obtained plant sources. Therefore, any product called Lauril should be a plant source. However, it is common to find “Petrochemical Lauril” sold on the market. Technically speaking, if the source of a product with a chain containing 12 carbons is petrochemical, it should be called Dodecyl and not Lauryl, but this is not the only difference.
The difference between these two sources is again in their solubility, with the petrochemical product generally being less soluble than the vegetable product. This can result in difficulty gaining viscosity and higher cloud points. Therefore, it is essential to know the source of obtaining it at the time of purchase and, as it is different the product constantly used, always test a sample for approval.
Having clarified these points, which often prevent us following a more assertive path, some relevant tips are also interesting to follow a clearer and safer path:
Choosing the thickener

The formula cannot perform well in terms of viscosity below a certain concentration of active ingredients, hence the need to use a thickener.
For a long time, two products were used to help add viscosity to detergents: Amide (Coconut Fatty Acid Diethanolamide) and Betaine (Cocostarch Propyl Betaine). However, these two molecules are not necessarily thickeners. They are great co-surfactants that greatly improve the quality of the foam and are emollients; that is, they protect the skin against the attack of active ingredients that cause dryness and dermatitis.
These two products do help to gain viscosity, but they should not be used excessively (maximum 0.5%) as they can create instability in the formulation as they increase the cloud point and make it difficult to rinse the product after cleaning. Sometimes, we repair colored stains on crockery and crystal glasses. These stains are generally caused by excess Amide and/or Betaine and indicate that the product was not completely rinsed.
There are several options for thickeners on the market, each type being more or less suitable for different formulations. Check them out below:
- Alkali soluble acrylics: This type of thickener is more appropriate for formulations with low active ingredients, the difficulty in adding viscosity is greater and the thickener options are fewer. In any case, the point of attention here is its oscillatory behavior concerning temperature. This type of thickener presents a very sudden drop in viscosity with increasing temperature and an abrupt increase in viscosity with decreasing temperature. So that this is not too impactful in the consumer's eyes, it is important to dose different amounts of thickener in summer and winter or for cold and hot regions. Otherwise, the product will behave very differently on both occasions (cold and hot). It is also important to ensure a quick turnover of the product on the shelf so that the formula that will be used in the summer does not remain on the shelf or in stock until the turn of the season and vice versa.
- Noxipon and similar ("synthetic starch"): This type of thickener is more suitable for medium and high active ranges (3 to 5%) and is exclusively associated with Sulfonic Acid. Therefore, low concentrations of Sulfonic Acid (less than 2%) do not allow good performance of this type of thickener. It is also not recommended to use concentrations greater than 3%, otherwise, the product tends to generate slight turbidity that does not cause precipitation but interferes with the appearance of the final product. Furthermore, there is no relationship between cost and benefit in higher dosages, as this cost could be used to increase the active content, which will also contribute to increased viscosity but will also increase cleaning and foaming performance.
- Cellulosic (CMC, HEC, HPMC): These thickeners possess significant thickening power but come with some aesthetic and technical limitations. Firstly, they tend to alter rheology Newtonian to non-Newtonian. In other words, they lose the characteristic of flowing smoothly, which is typical for consumers, and instead begin to exhibit a "slime" consistency that users do not particularly appreciate. Due to the molecular characteristics, these products often adhere to surfaces, making rinsing challenging. Therefore, they should be used at the lowest possible concentrations (maximum 0.1%). Finally, there are challenges related to the dilution process, as it is quite common to require pre-dispersion of the product in hot water before incorporating the thickener into the formula, depending on the tank's agitation system. The greater the shear capacity of the agitation system, the less pre-dispersion is necessary.
Essence, colorings, preservatives, and the use of a good sequestrant
These ingredients tend to suffer oxidation processes. One of the factors that most trigger oxidative processes in cleaning products and that directly interferes with these three ingredients is the presence of iron in the process water.
For this reason, it is very important to check the presence of iron in the water used for manufacturing very often. This periodicity will depend very much on the source of the water, whether it is well water, drinking water, river water, etc. The greater the chance of contamination, the more frequent the checks should be, and it may even be necessary to check daily or even twice a day.
Another important action to protect the formula iron is the addition of a small percentage of sequestrant. The sequestrant should not correct problems with high concentrations of contamination but should be added as a preventative measure against possible contamination that gets out of hand.
One of the most used sequestrants for this purpose is EDTA, with a concentration of 0.05% that should be sufficient for this purpose. However, EDTA has been heavily criticized due to its resistance to biodegradability. In this regard, it is more advisable to replace this ingredient with Dissolvine GL 47-S, a sequestrant with very similar performance that is 100% plant-based and rapidly biodegradable. Click here to find out more about this solution.
If you want more in-depth information, Macler's blog has an article for each of these ingredients: dye, fragrance, and sequestering.
The secret is synergy!
Traditional detergent formulations, composed of the active ingredients Sulfonic Acid and Sodium Lauryl Ether Sulfate, do not seem to give us much room for differentiation in for this reason, many formulators stick to the concentration of active ingredients, believing that this is the only way to increase performance.
However, high efficiency can be achieved if synergistic relationships between active ingredients are identified. An optimum relationship between active ingredients allows high performance to be achieved with lower dosages of these ingredients.
This is what synergy is: when the sum of the parts generates a result that exceeds its isolated parts. It's the famous 2 + 2 = 5.
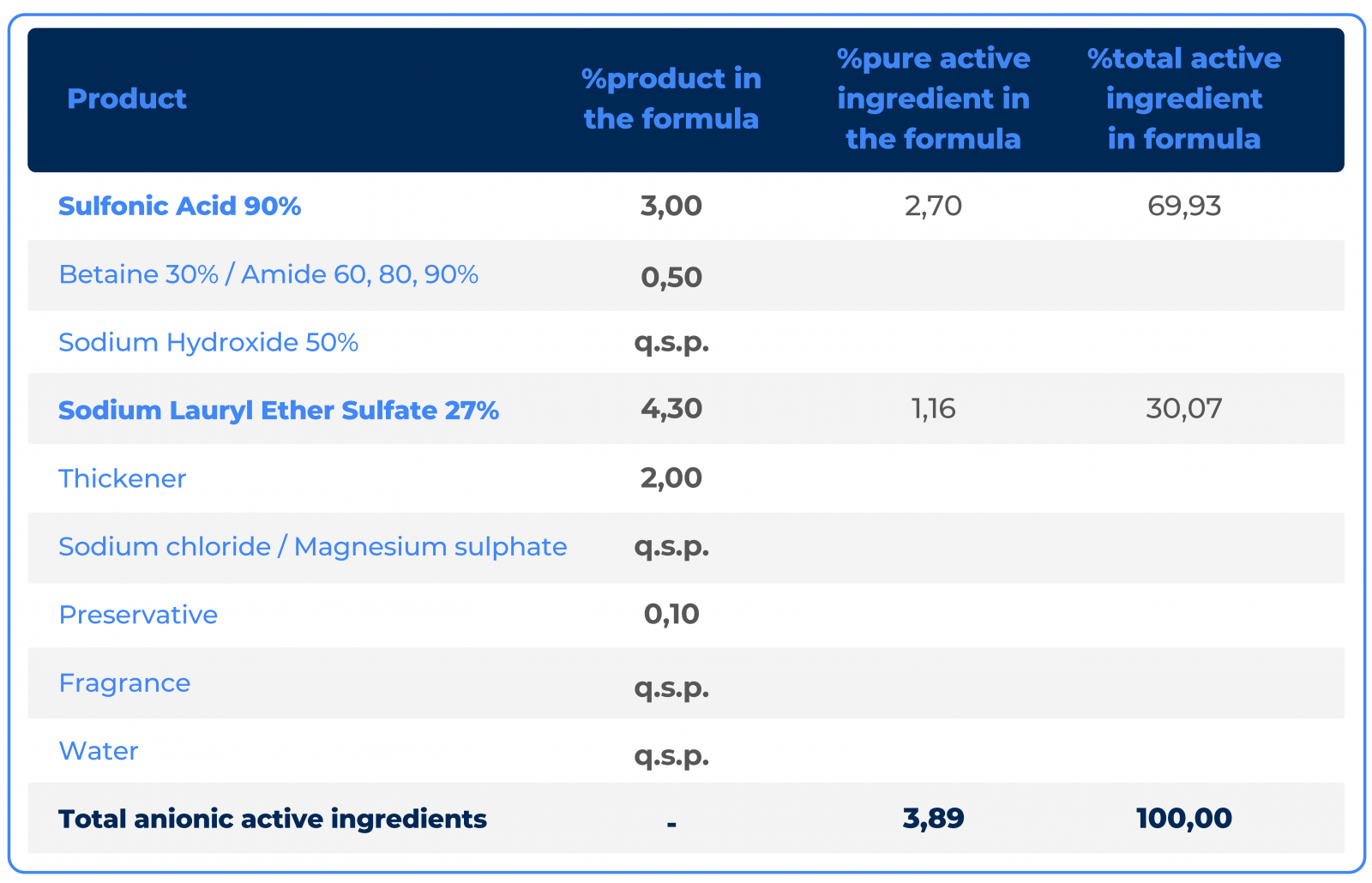
This type of investigation into synergistic relationships between active ingredients has been one of Macler's main research themes for over 20 years. Among these various accumulated studies, it is already known that the best relationship between Sulfonic Acid and Sodium Lauryl Ether Sulfate is between 65% Sulfonic Acid and 35% Sodium Lauryl Ether Sulfate, and 80% Sulfonic Acid and 20% Sodium Lauryl Ether Sulfate.
This calculation only takes into account pure active ingredients, and their dosages must be converted according to their concentrations,according the examples in the table below:
Through all this time, searching for knowledge and investigating synergies, Macler was able to develop a fine synergistic relationship of high performance between various active ingredients that culminated in Isogen PRO-D. Click here to find out more about this product.
With Isogen PRO-D, development work becomes extremely simple and efficient. In addition to all the reduction in effort to develop an efficient, differentiated, and competitive formula, Isogen PRO-D also drastically reduces process time, as it does not require neutralization and can be pumped directly into the tank, also reducing the number of ingredients to be added.
With the appropriate process, Isogen PRO-D can double production capacity.
If you have reached this stage in your reading and can absorb all this content, it will be easy to use the detergent formulation guide below:
Remember: the SmartLab team is on hand to provide the support you need to make your formulation a success.
Learn More
Perhaps one of the best-known chemicals used by Brazilian consumers, which has a dramatic influence on culture and behavior with regard to cleaning, is sodium hypochlorite, commonly known as “bleach.”
When the temperature changes, turbidity, phase separation, crystal formation, or changes in viscosity may occur. Two parameters are critical in this analysis: the fog point and the cloud point.
This article will discuss best practices for peroxide-based formulations and tips for developing a high-performance, cost-competitive, and highly stable product.
Our chemistry
We use our labs to create
intelligent chemical solutions balanced with your reality.
Products
We use our R&D center, our own laboratory with experienced professionals, to deliver intelligent chemical solutions in balance with your reality.

