
Hydrotropes: what they are and how to choose the best one
When developing products, many of the challenges we face have to do with solubility. Products separate, cloud, crystallize, or emulsions separate or are not completely translucent.
On several occasions, it is not possible to adjust the formula simply by changing the relationships between the components. In these cases, it is necessary to add some ingredient that solves this problem, which can be a solvent such as an alcohol or a glycol, for example. In the overwhelming majority of cases, the best option is to use a good hydrotrope.
But what is a hydrotrope?
In simple terms, they are products capable of increasing the solubility of the organic compounds in a mixture. In this context, they increase the solubility of surfactants.
In the hygiene and cleaning or cosmetics markets, the most commonly used and known are Sodium Xylene Sulfonate, Sodium Cumene Sulfonate, and Butyl Glycol.
However, it is worth considering that there is a great diversity of hydrotropes that present better efficiency. Each system and each need will require a specific hydrotrope nature.
In this article, we will exemplify some classic cases of formulation instability that are generally more critical, in order to present options for very efficient hydrotropes that can be your allies in product research and development.
Situation 1: Products with high Sulfonic Acid concentration
When the product has and requires a high concentration of Sulfuric Acid, it is common for it to become unstable, cloudy and separate easily. Especially if 96% Sulfuric Acid is used, which is less soluble compared to 90%.
Check out the hypothetical situation below:

The application of different hydrotropes was carried out with the aim of lowering the cloud point to 10°C. See the result obtained below:
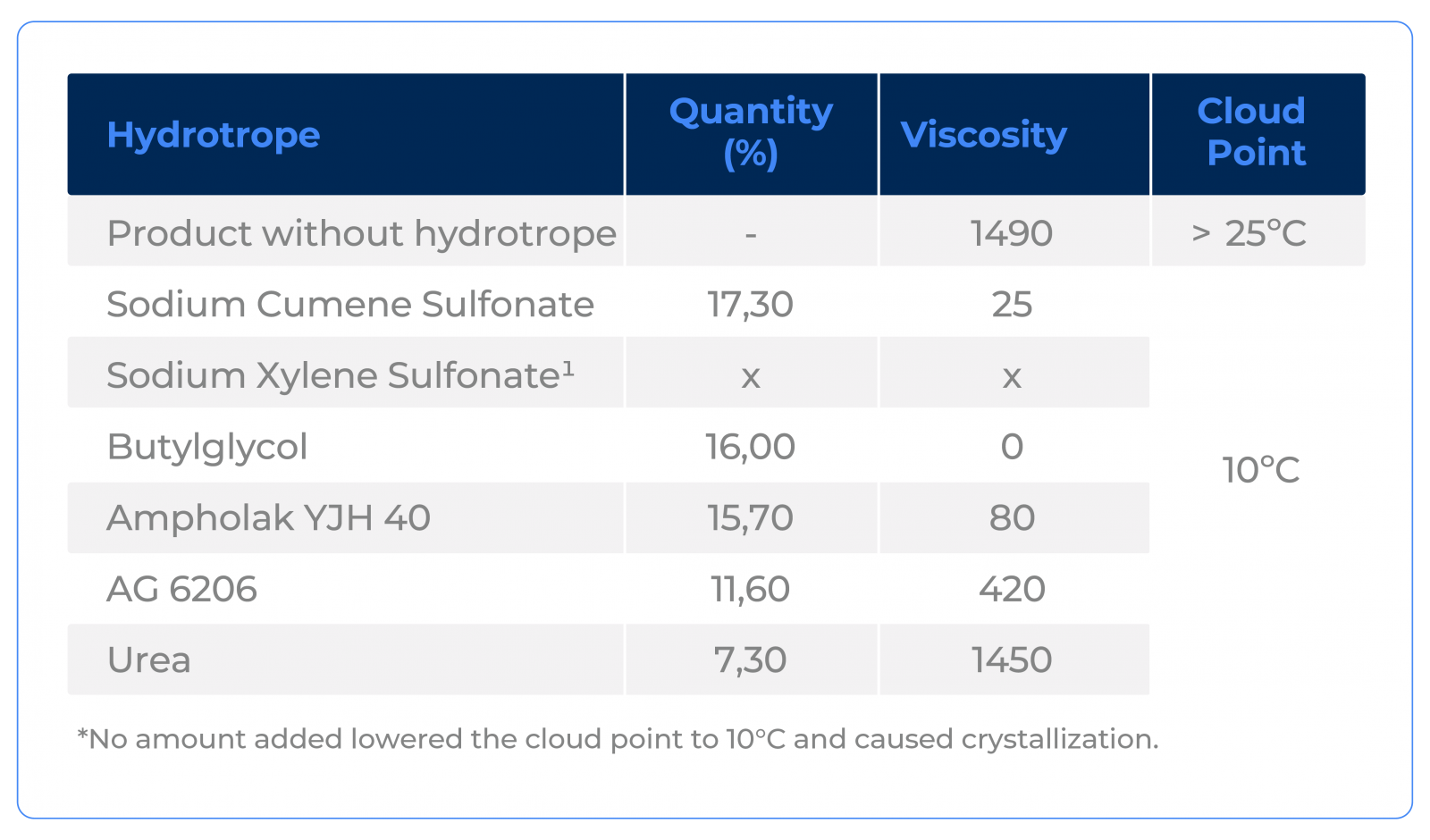
Notice that Urea was the one that presented the lowest concentration necessary to reach the desired cloud point.
In cases of formulations of this nature, Urea is, in general, the most economical option. In addition to being the one that demands the lowest concentration, it is, by far, the cheapest option.
Note that, in addition to greatly improving solubility with the best cost-benefit ratio, it was also the one that least affected viscosity. If this is a limitation, Urea still has this advantage. If you want to break the viscosity, a mixture of two hydrotropes can be used. The combination of using Urea with Butyl Glycol or Ampholak YJH 40 can be a good option.
The fact that Urea has less impact on viscosity is due to the fact that it is the only one of the options tested that tends not to interfere with the micellar arrangement of Sulfuric Acid, as it acts on the solubility of the system, interacting with the medium, but without interacting with the surfactant in such a way to become part of the micelle.
.png) For this system, there is no doubt that Urea is the best option.
For this system, there is no doubt that Urea is the best option.
Situation 2: Emulsions
Another very common situation is the correction of various emulsions. With a suitable hydrotrope, it is possible to transform opaque emulsions into microemulsions. Microemulsions generate very stable final products with a translucent appearance.
To exemplify this situation, consider the hypothetical formulation of an industrial degreaser:

We add again some hydrotrope options, but seeking to increase the cloud point to 50°C.
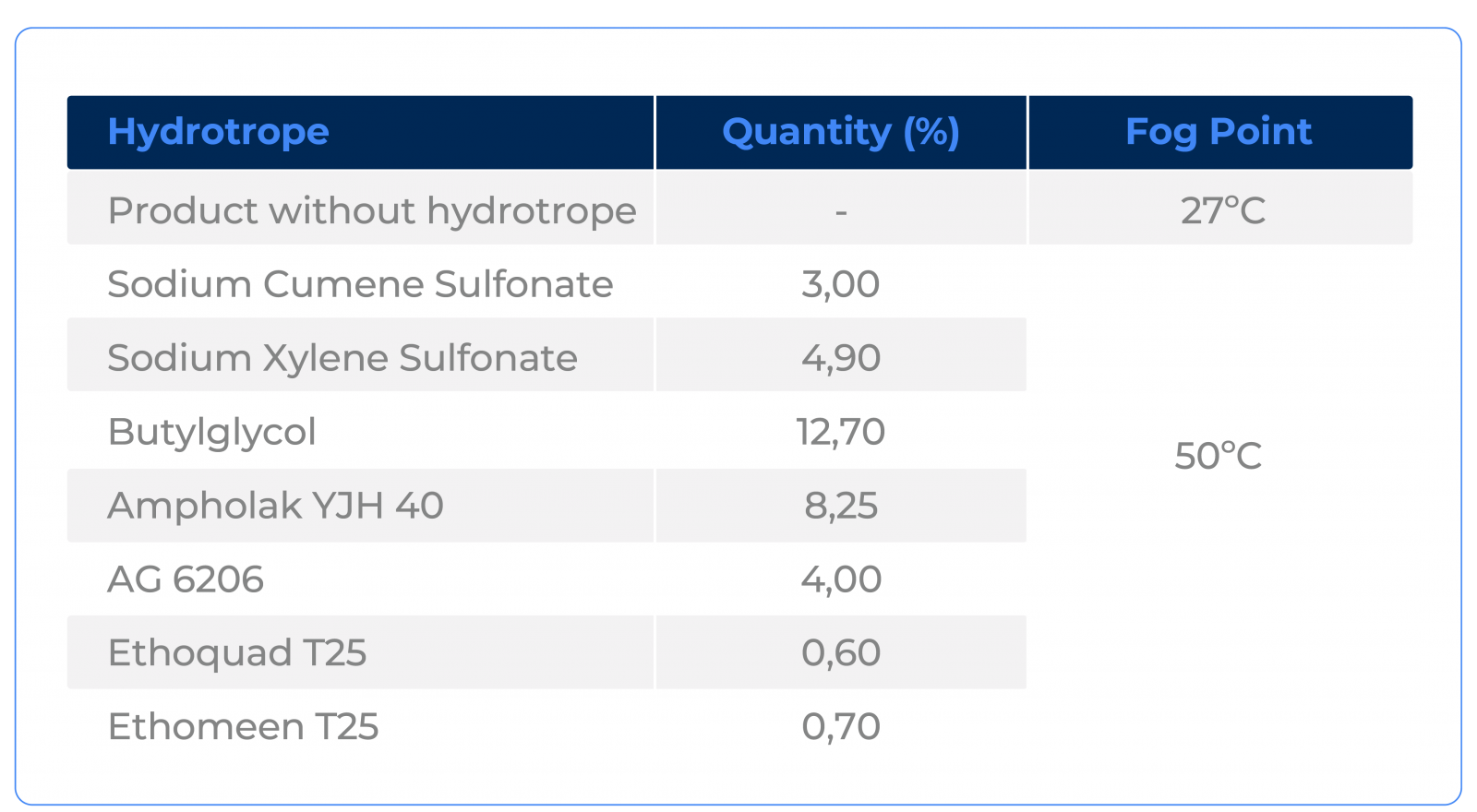
Notice that, again, the test results with the best-known hydrotropes are not necessarily the ones that present the best efficiency and cost-benefit ratio.
On the other hand, it is observed that Ethomeen T25 and Ethoquad T25 are excellent options. In this type of system, it is interesting to note that the best results are achieved when hydrotropes with low ionic activity are used, but with a larger apolar chain.
Ethomeen T25 and Ethoquad T25 are ethoxylated fatty amines which, compared to Sodium Xylene Sulfonate and Sodium Cumene Sulfonate, have lower electrolytic strength and larger chains. Since electrolytes tend to destabilize emulsions, the tendency is for low ionic strength hydrotropes to be more effective.
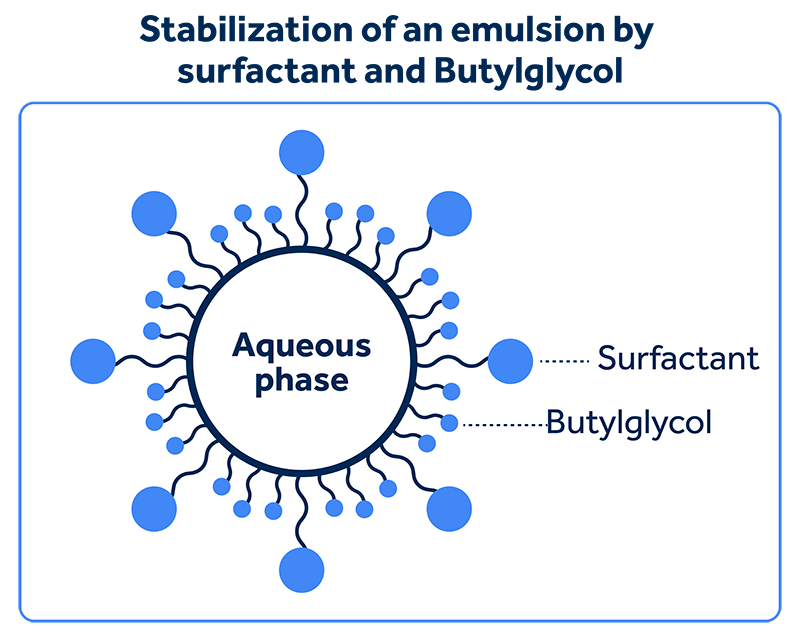
All the tested hydrotropes interact with the micelles of the surfactants in this system, forming a mixed micelle which, due to containing a “larger” polar head, makes the micellar system more soluble, without necessarily losing its efficiency as an emulsifier.
.png)
As the polar head of Ethomeen T25 and Ethoquad T25 comes their high degree of ethoxylation, a large, but non-ionic and, consequently, less electrolytic polar head is formed.
Meanwhile, Sodium Xylene Sulfonate and Sodium Cumene Sulfonate have large, but electrolytic polar heads. Butyl Glycol has a very short carbon chain and polar head, and for this reason, it demands high concentrations to be able to regulate the solubility of the medium.
Situation 3 - Strongly caustic formulations
A very extreme situation, but very common in professional products, are formulations with very high concentrations of caustic soda.
Consider the formulation below as an example:

Very few hydrotropes have stability in this type of drastic system. Notice that, in all the tests carried out, only two hydrotropes were able to dissolve and help stabilize the system.
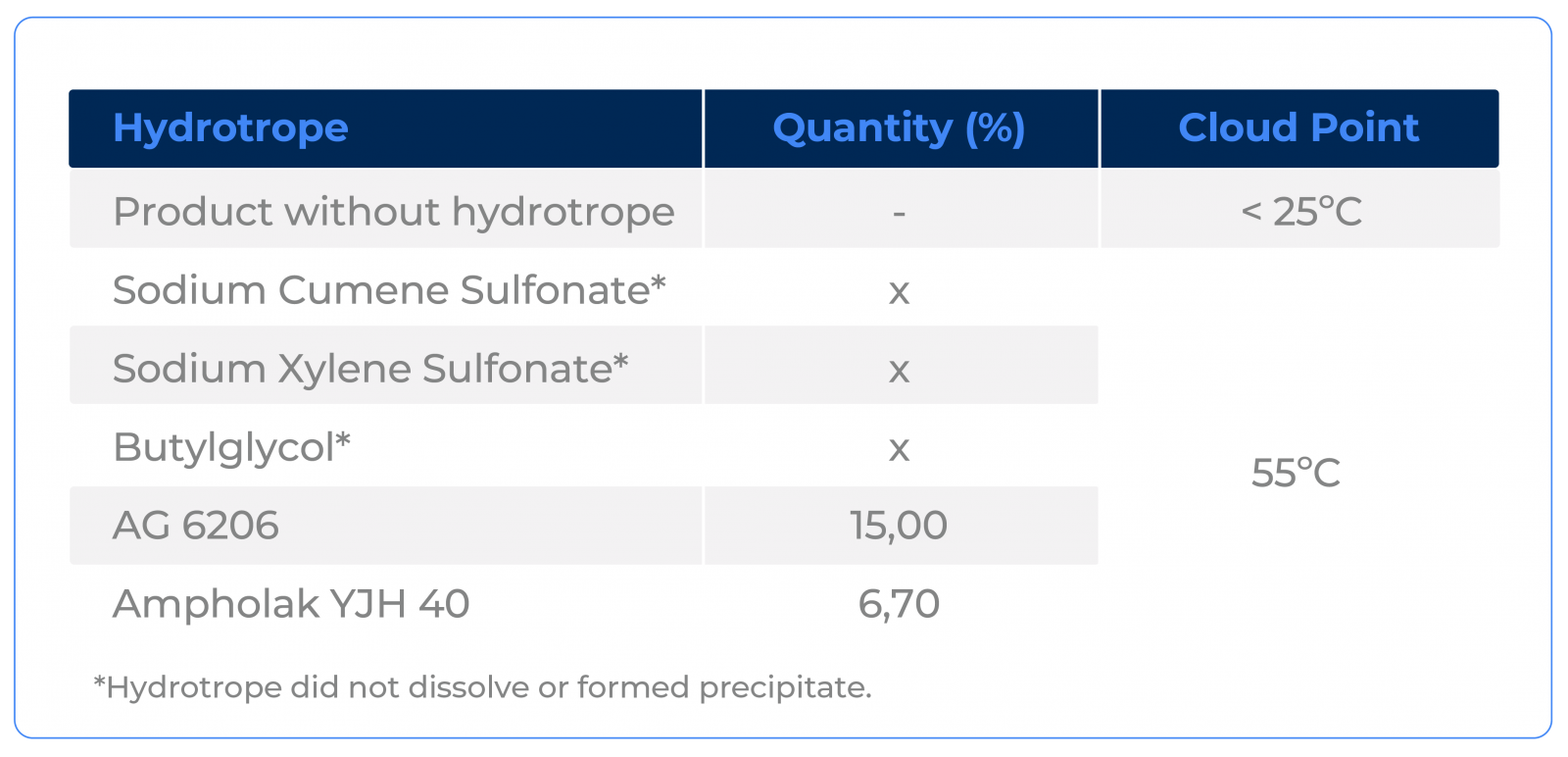
This is a condition in which we have not found a better hydrotrope option than Ampholak YJH 40, a product manufactured by Nouryon and distributed by Macler. Note that only Ampholak YJH 40 and AG 6206, another product manufactured by Nouryon, were able to stabilize the formula. Even so, the dosage of Ampholak YJH 40 was considerably lower, presenting a better cost-benefit ratio.
In this case, Sodium Xylene Sulfonate and Sodium Cumene Sulfonate did not dilute and Butyl Glycol ended up behaving like an oil due to the very high electrolytic strength of the system, as expected.
In general, in systems that are not so caustic, Macler usually solves solubility problems only by making adjustments between the components or suggesting alternative actives, aiming at maintaining or reducing the formula cost, without the need to add hydrotropes, which usually add costs.
Points of attention when choosing a hydrotrope
Although some hydrotropes may present better or worse performance in the cases presented previously, it is worth clarifying some points of attention when choosing the appropriate hydrotrope for the desired functionality of the final product.
In addition to its hydrotropic power, some components present greater or lesser foaming. Check the foam volume and collapse of each hydrotrope used in the situations above:
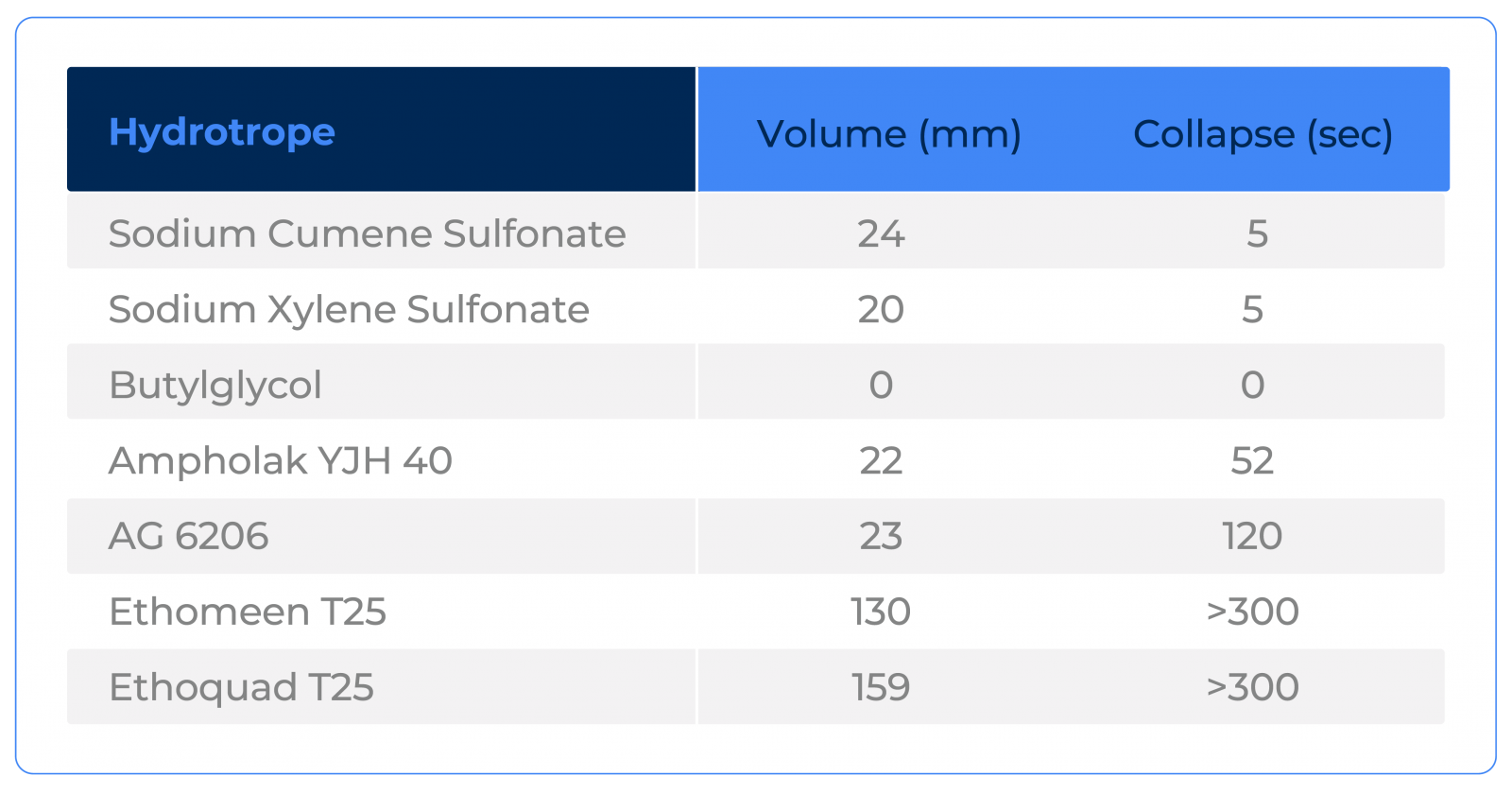
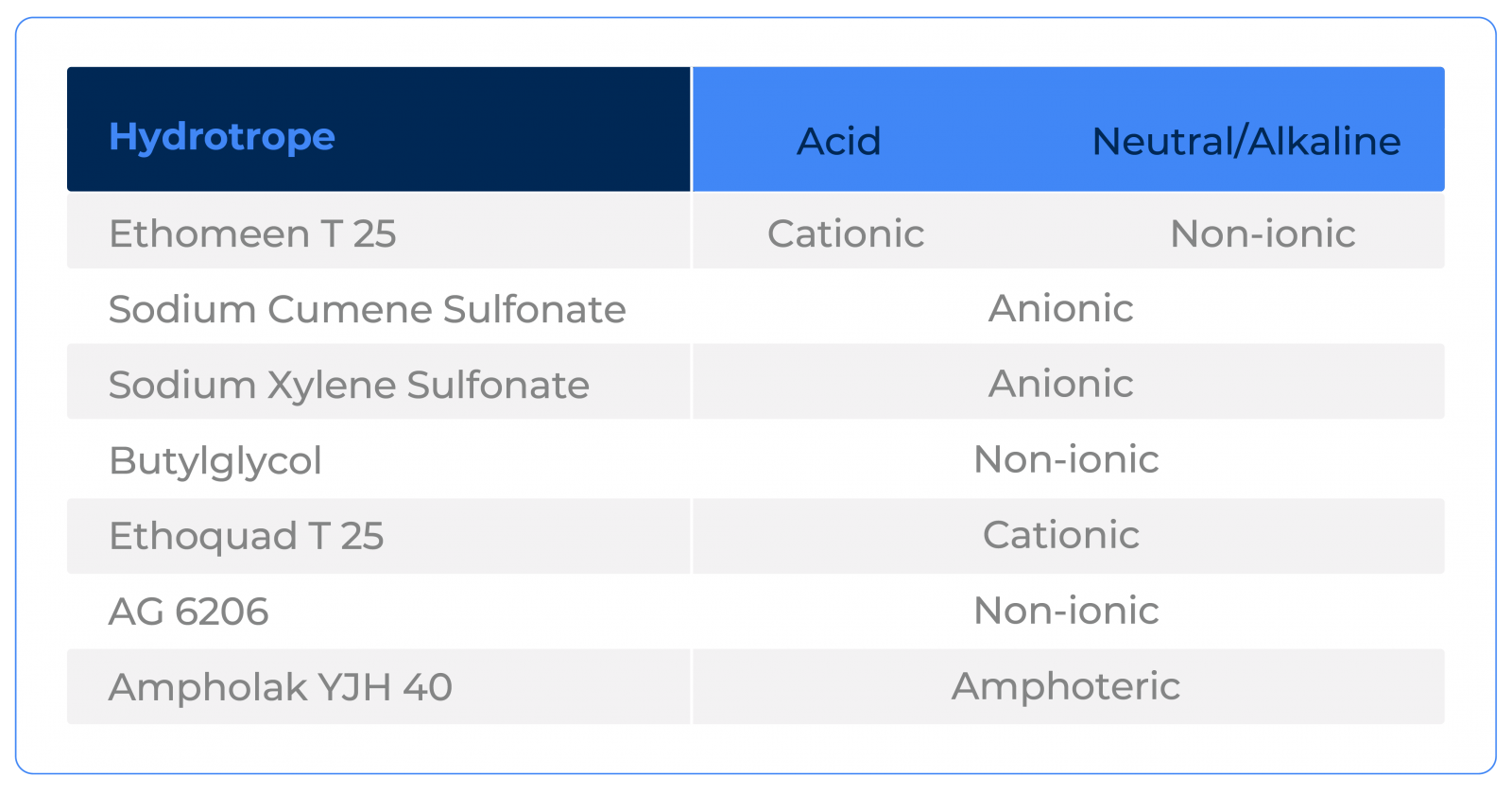
Another important factor to pay attention to is the ionic character of the hydrotrope. Depending on this, it may not be possible to use it for certain systems. For this reason, Ethoquad T25 was not tested together with Sulfuric Acid, for example.
Cationic products tend to adhere to the surface and, therefore, can both present a benefit, such as adding anti-corrosive characteristics, or be a limitation, as depending on the system, they can generate the feeling of "tac" on the surface.
Closing with tips
In conclusion, check out a summary with tips to consider when choosing a hydrotrope:
- It is unlikely that the best-known hydrotropes, which have a lower price per kilo, will present the best cost-benefit ratio. Try testing alternatives with technologies suitable for each system. At the end of the tests, it is possible to notice that, in general, "cheap is expensive" and options that previously seemed expensive are actually those that will bring greater savings.
- In systems with a high concentration of Sulfuric Acid, start by testing Urea, it is unlikely that a better cost-benefit ratio will be found. But be aware! In alkaline systems, Urea degrades to form ammonia, which produces an unpleasant odor.
- In emulsion systems, look for hydrotropes with low conductivity/electrolytic strength, such as Ethomeen T25 and Ethoquad T25, the ethoxylated fatty amines we used in the tests presented in this article.
- In systems with high causticity, that is, with high concentrations of Caustic Soda, test Ampholak YJH 40. If you want lower foaming levels, we suggest using AG 6206.
- Regardless of the system, do not forget to take into account the desired foaming levels and the impact of the hydrotrope's ionic characteristic on the final product.
.jpg)
Whatever the situation you are working on, contact our SmartLab and find out how we can help you choose the best hydrotrope for your case!
Count on Macler as your strategic chemical partner in the most challenging moments!

Learn More
Perhaps one of the best-known chemicals used by Brazilian consumers, which has a dramatic influence on culture and behavior with regard to cleaning, is sodium hypochlorite, commonly known as “bleach.”
When the temperature changes, turbidity, phase separation, crystal formation, or changes in viscosity may occur. Two parameters are critical in this analysis: the fog point and the cloud point.
This article will discuss best practices for peroxide-based formulations and tips for developing a high-performance, cost-competitive, and highly stable product.
Our chemistry
We use our labs to create
intelligent chemical solutions balanced with your reality.
Products
We use our R&D center, our own laboratory with experienced professionals, to deliver intelligent chemical solutions in balance with your reality.
